Dry eye disease (DED) is a complex condition with many contributing factors. But what if you could quickly and confidently diagnose and subtype it for your patients?
Look no further than the Medmont Meridia™. It offers gold-standard topography and robust dry eye disease assessment capabilities, providing you with invaluable insights to guide the right treatment path.
Let’s run through the Meridia™’s dry eye modules designed to make your job easier.
Dry eye workflow by Dr Aaron Wolf
Anterior imaging and video
The Meridia™’s HD colour camera lets you capture detailed photographs and video with little effort for robust pathology documentation.
When it comes to dry eye disease, its anterior imaging with white light allows you to confidently evaluate redness and inflammation, blepharitis, eyelid hygiene, meibomian gland expression, blinking and more.
It also offers a consistently sharp depth of field and a large field of view—with illumination across the entire surface, so you can see more at a glance. In contrast, slit lamps have a single source of light which can cause shadowing on the opposing side.
The result? Highly detailed photographs to refer to when tracking changes or disease progression. They’re invaluable as supporting documentation when referring patients, aiding continuum of care.
Show the crisp images and video to patients to help them visualise what you’re explaining and to improve compliance.
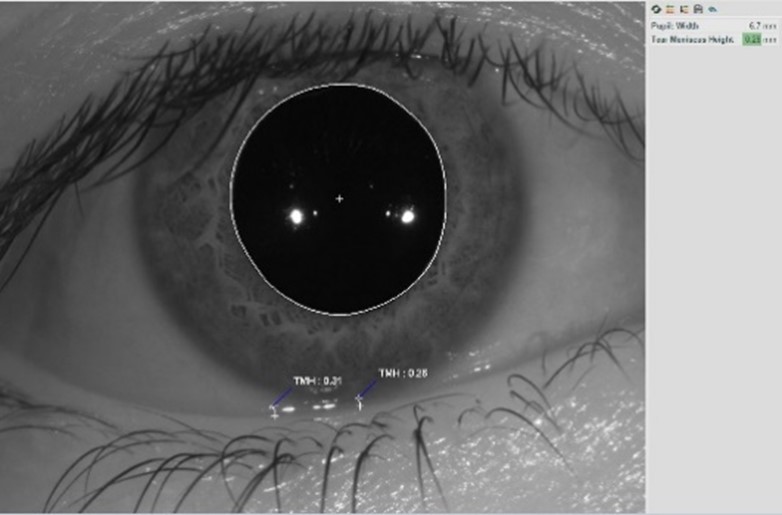
Tear meniscus height measurement
Previously, you had to use caliper tools or unconventional methods to gain this contributing piece of evidence for subtyping dry eye. A gross estimate makes things harder in an already challenging environment.
The Meridia™’s tear meniscus height (TMH) ruler allows you to quickly and accurately gain a quantitative tear volume measurement for an important piece of the subtyping puzzle with minimal effort.
What’s more, you can use the TMH annotation tool on any existing anterior image or topography capture, saving you time with one less step in your workflow.
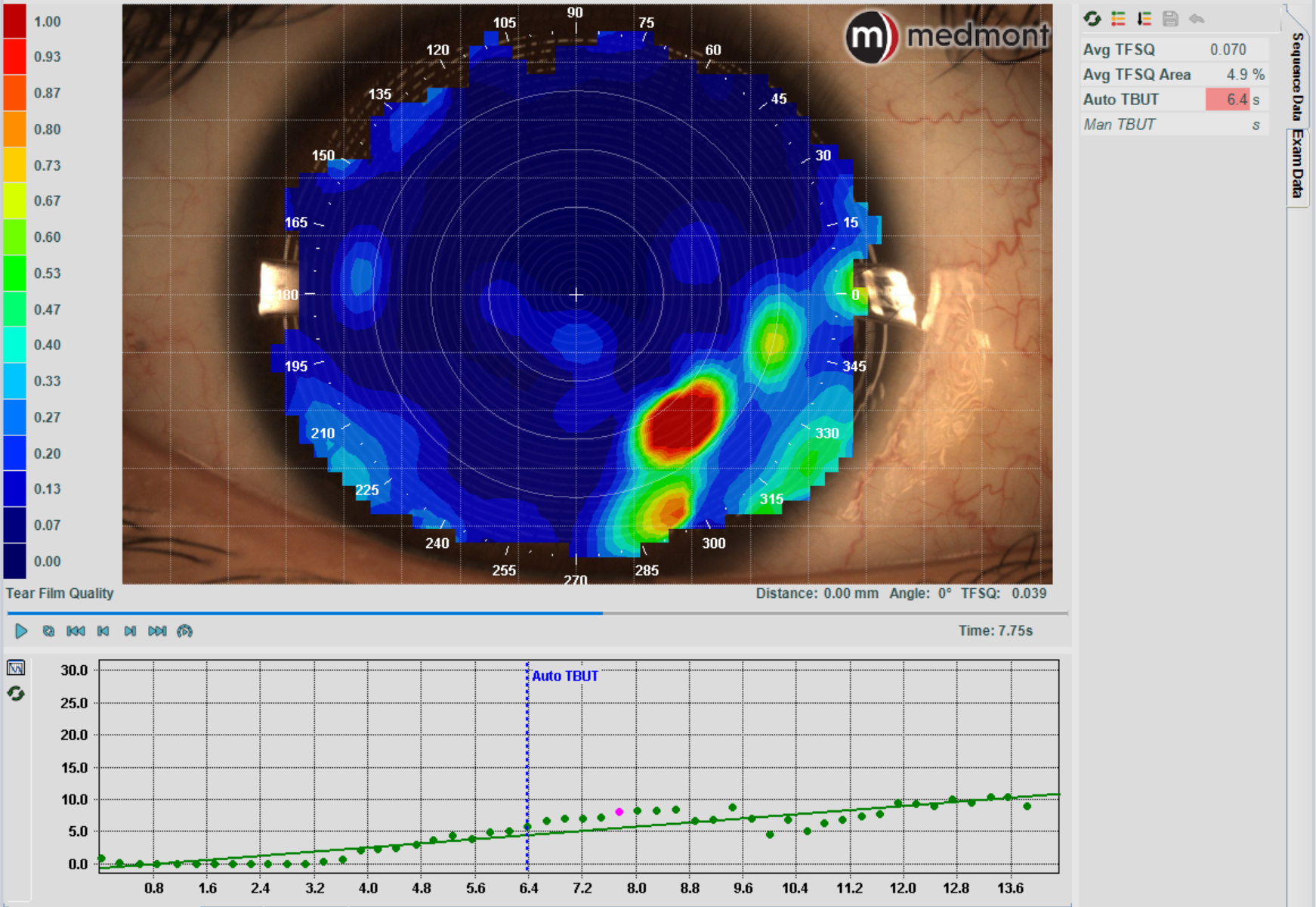
Tear film analysis mode
A Tear Break Up Time test (TBUT) assesses tear film stability and allows you to check for evaporative dry eye. It measures the time it takes for the tear film to break up after a blink.
While this test is traditionally performed with sodium fluorescein dye, research shows it’s more accurate without it. The Meridia™ offers a non-invasive alternative (non-invasive tear break up time or ‘NIBUT’) measured in seconds to 1 decimal place, as well as a map of the tear film surface quality (TFSQ) so you can identify the location of unstable tear film on the cornea.


Meibomian gland imaging
For another key piece of the DED sub-typing puzzle, it’s essential to visualise and assess the state of the upper and lower meibomian glands.
Using infrared light, the Meridia™’s meibography function allows you to take high-definition images of the meibomian glands in the upper and lower lids, so you can screen, track and document Meibomian Gland Dysfunction (MGD), including any future gland loss or dropout.
Conveniently, the Meridia™ has a smaller head than other topographers and the working distance from the patient is shorter. That means a single operator can easily reach over to hold the patient lids and capture images at the same time.
MGD focused solutions are some of the highest revenue-generating treatments, so it’s important to show your patients why they’re worth the investment. Tracking glands with images lets you demonstrate the need for treatment, show how a treatment is working or emphasise disease progression to non-complying patients.
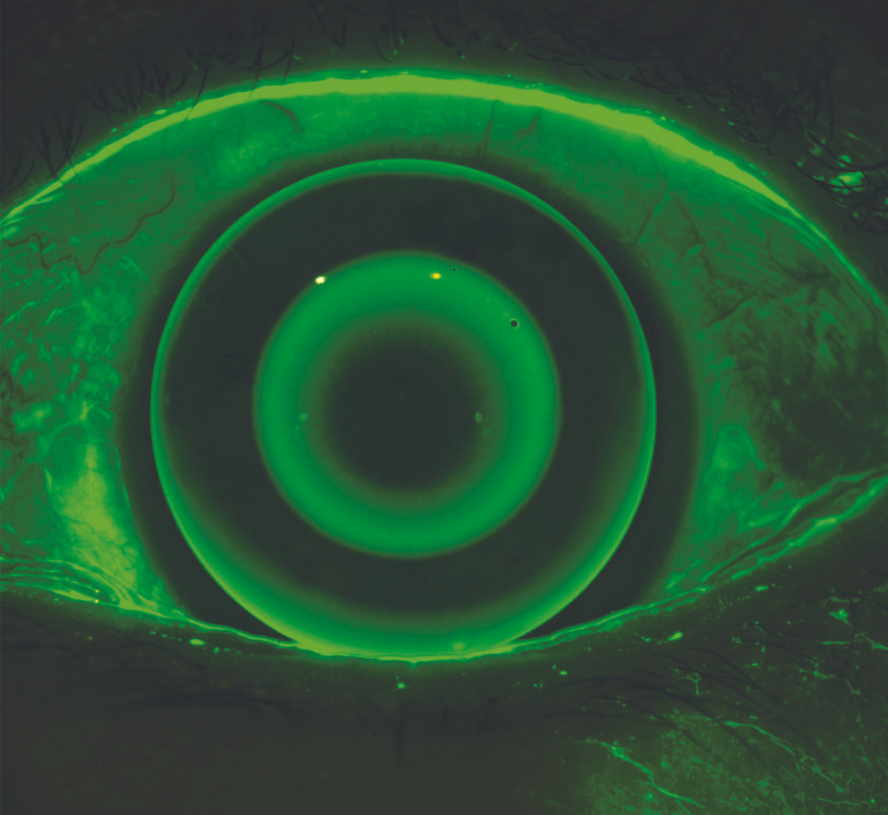
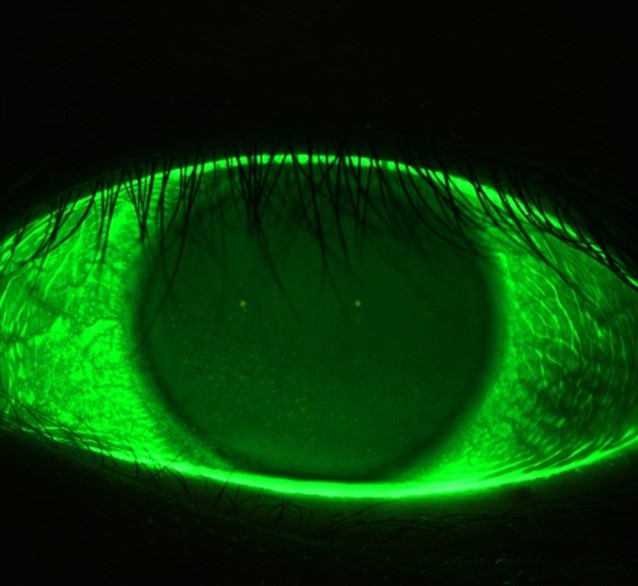
Fluorescein imaging and video
You can assess the condition of the anterior surface with the Meridia™’s fluorescein imaging and video. This exam module activates cobalt light to highlight any conjunctival or corneal staining, in addition to aiding tear film assessment.
This feature allows you to visualise and document corneal surface complications with images of exceptional clarity—in some cases, without the need for a slit lamp in your workflow.
Standardised dry eye grading scales
It’s difficult to track changes over time if you grade ocular conditions without standardised scales. And if all the practitioners in your practice use their preferred way to grade, your patients will receive different information. Cue confusion.
By using the Meridia™’s included standardised dry eye scales (Efron, BHVI, Meiboscale) to grade images, you’re ensuring gradings are reliably universal.
Use these gradings to educate patients on their level of disease and monitor outcomes to inform treatment plans. In doing so, you can be more confident in your clinical decisions.
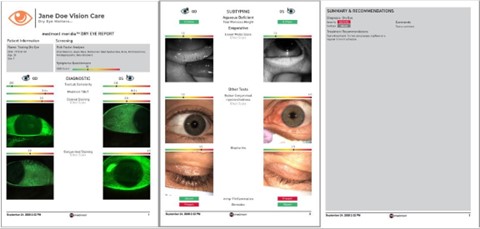
Comprehensive dry eye reports
The Meridia™’s dry eye report function quickly pulls selected tests and images in your dry eye workflow into one visual report, complete with grading scales.
Print the report to confidently present your findings to your patient in a visual way, helping them understand your diagnosis and why they should comply with treatment suggestions. Generate a new report at each visit to compare and track treatment impact over time. It truly makes the management plan and sales process easier. And the results will speak for themselves.
Would you like to know more?
It’s clear that the Medmont Meridia™ is a powerful, multi-functional instrument that adds immense value to your practice and business.
It offers robust dry eye imaging capabilities to:
- inform dry eye disease diagnosis and subtyping,
- improve patient education and compliance, and
- guide treatment strategies for better patient outcomes.
And this article hasn’t even touched on its outstanding topography capabilities.
Contact us to find out how the Meridia™ can benefit your practice.
For practical information on how to subtype Dry Eye Disease, we recommend referring to the TFOS DEWS II report, The Ocular Surface (2017).
To learn more, fill in the form below to download your free Medmont Meridia™ feature guide.
